

Beauty Lies Just Beyond The Surface.
Vivace Ultra™ combines two unique technologies into one personalized treatment, highlighting the movement towards graceful, healthy aging and skinimalism. By offering industry-first ultrasound visualization with unmatched precision, Vivace Ultra™ is the most recognized radiofrequency (RF) microneedling experience emphasizing the importance of colorblind versatility and beyond the surface tailored treatment plans.
Learn More

Experience Vivace. Experience The Results.™
The innovators at Aesthetics Biomedical® have designed the Vivace Experience® an exciting, best-in-class, proprietary treatment. We start with the Vivace®, a best-in-class RF microneedling device for incomparable patient experience.
Learn More

Complexity of Science. Simplicity of Skincare.
Aesthetics Biomedical® Inc. has launched a personalized topical product line for take-home use. SoME® Skincare is a skin rejuvenating product that uses a propriety blend of ingredients combined with your own Platelet Rich Plasma (PRP), by your physician, to give you a truly personalized cosmetic experience.
Learn More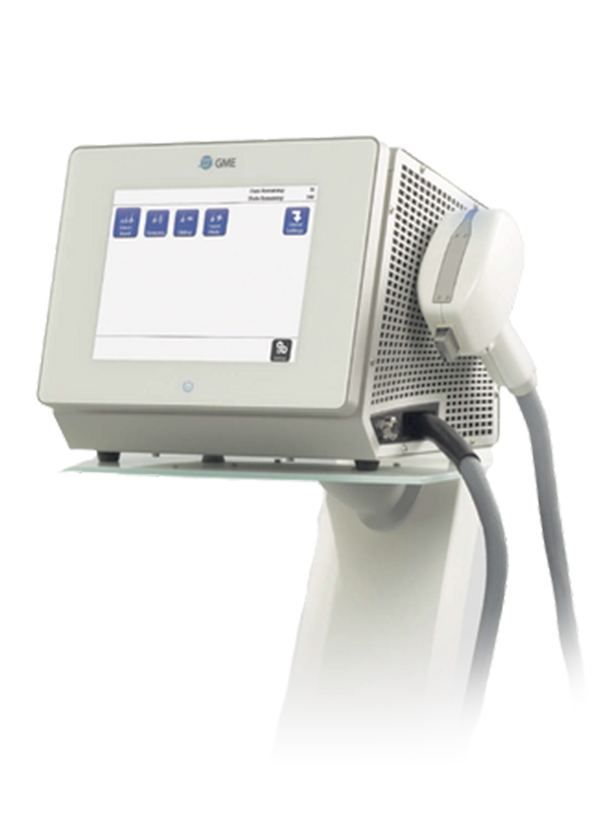

Be Gone. Be Good. Be Fast.
Enjoy the experience of virtually pain-free hair removal. A high-powered 8o8nm diode laser for virtually pain-free permanent hair reduction that treats ALL skin types. The SENZA Experience is fast. A medium sized area can be treated in as little as five minutes with a full back completed in ten minutes.

Jennifer Saviano, ABC’s The Bachelor & Bachelor in Paradise
“There is something about Vivace® that is special – the downtime is significantly less than regular microneedling. After multiple treatments, I feel like my makeup just glides over my skin.
I like to call it the Benjamin Button facial. It’s literally my go-to treatment.”
Awards & Press










Advisory Boards
Learn MoreClinical Data
Learn MoreBeauty Press
Learn MoreDigital Events
Learn MoreStore
Learn MoreLive Events & Tradeshows
Learn MoreMission Statement
Aesthetics Biomedical®, Inc., headquartered in Phoenix, Ariz., is committed to the development and distribution of novel aesthetic devices, products, and services in the global market. Aesthetics Biomedical’s innovation center is a leader in breakthrough technologies and combination therapies for its clients, physician network and the aesthetic arena, creating novel patient treatment experiences that benefit from ongoing research, approved clinical indications for use, as well as a personalized approach designed for consumer benefit.

